Week in Washington is brought to you by Michael Cohen, PhD. Tune in each week to read the latest on healthcare policy and get a glimpse of what’s on the horizon.
Week in Washington
10/29/20
A busy week in Washington.
SCOTUS – On Monday, Amy Coney Barrett was confirmed to the Supreme Court. She takes her seat immediately and is expected to take part in many of the upcoming key health care cases that involve ACA constitutionality and Medicaid Work Requirements.
Congress – With the approval of Barrett, the Senate went on recess. It (and the House) are not generally expected to reconvene until later in November. This means the potential for a COVID stimulus agreement will not occur before the election and generally is unlikely at least until December.
COVID
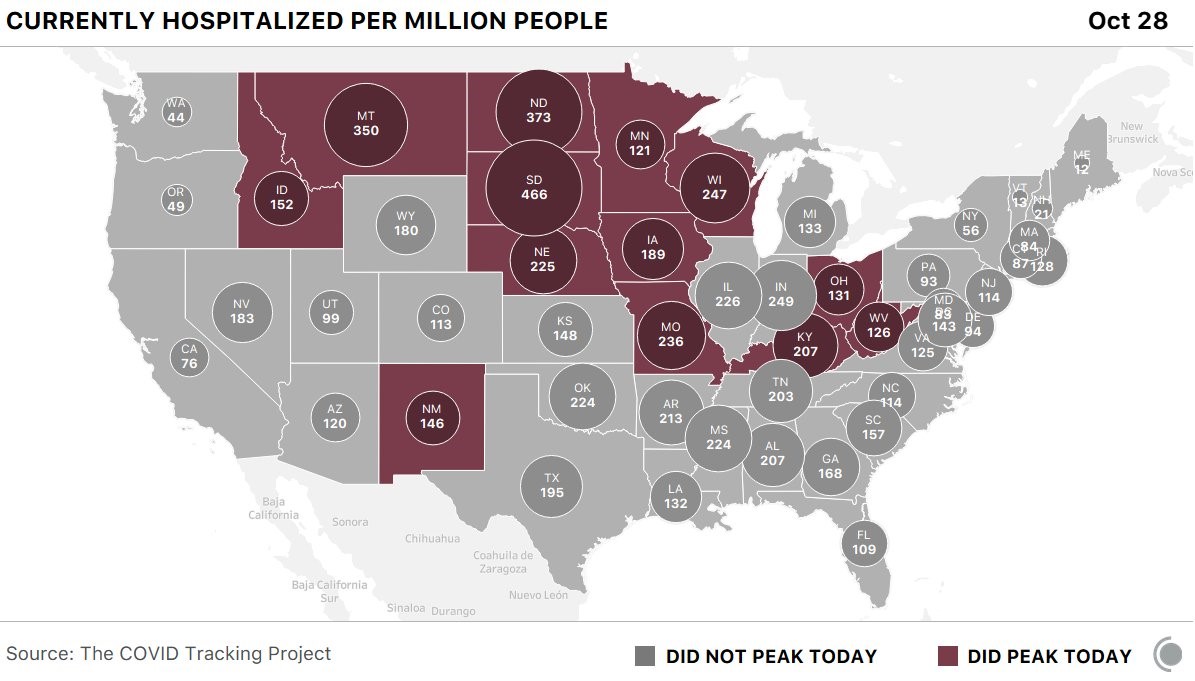
Unfortunately, the number of COVID cases continues to escalate. The seven-day average of daily cases hit an all-time high this week (around 75,000 cases a day), and hospitalizations also significantly increased (currently averaging about 40,000). As you can see below, the Dakotas and Montana have, by far, the highest rate of hospitalizations per capita in the country currently.
Unemployment
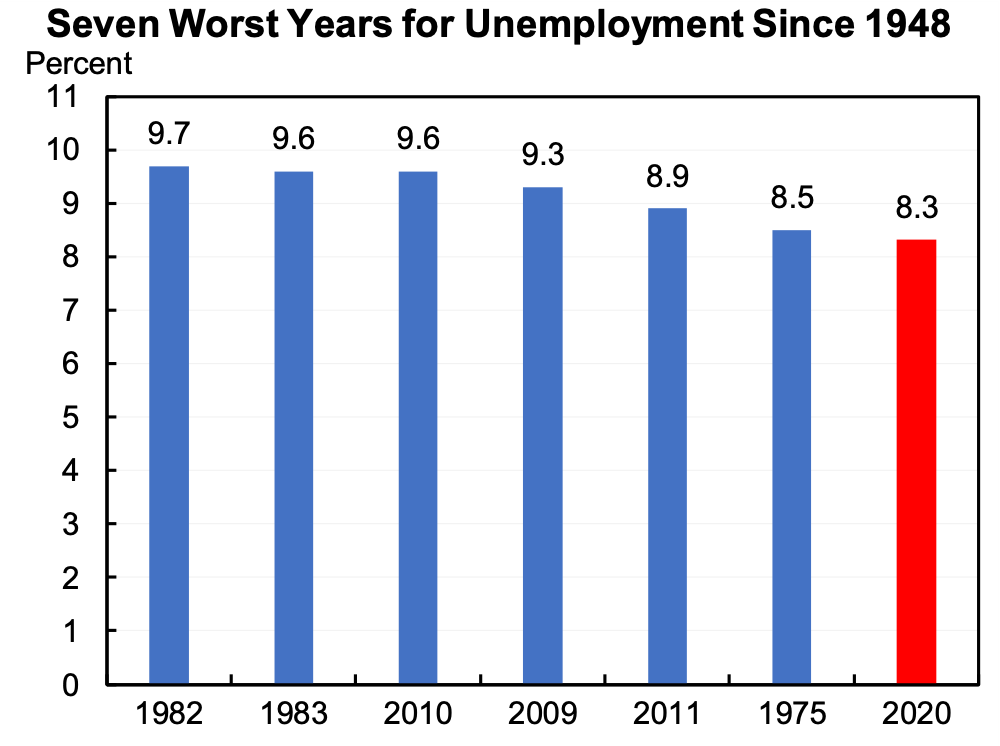
Based on the recent GDP numbers and recent unemployment, Jason Furman placed the current unemployment numbers in context to previous economic downturns, marking this year the 7th worse since 1948.
CMS Regulations
CMS released two major regulations this week.
Transparency Regulation – CMS finalized its transparency regulation. The regulation would require most non-grandfathered group health plans offering coverage in the individual market to make available out of pocket information and underlying negotiated rates. They will also make available detailed pricing information. Insurers will need to provide information on 500 shoppable services starting in 2023 and all health care information starting in 2024. You can read the rule here.
COVID Related Regulation – CMS also released an interim final rule (with comments) on additional COVID items. The rule made clear that once the FDA approved a COVID vaccine, even under an EUA, that the vaccine would be covered under Medicare Part B without coinsurance or deductible. CMS will announce coding and payments on the vaccine in the future. The rule also provided states with flexibility to end optional benefits for their Medicaid programs without the risk of losing access to the increased FMAP payments. Finally, the rule required most private health insurers to cover a COVID vaccine during a public health emergency. You can read more about the rule here.
Reminder: The election is Tuesday. We’ll be covering some implications in the next blog.
Previous editions:
10/22/2020: Week in Washington
10/15/2020: Week in Washington
10/08/2020: Week in Washington
10//01/2020: Week in Washington
09/24/2020: Week in Washington
09/17/2020: Week in Washington
09/10/2020: Week in Washington
09/03/2020: Week in Washington